
OneClass: For this reaction: MgSO4 (aq)(NH4)2CO3(aq)> What one of the following species would appe...

How many grams of hydrogen are there in 74.11 g of (nh4)2co3? Hint: the molar mass of (nh4)2co3 is 96.11 - Brainly.in
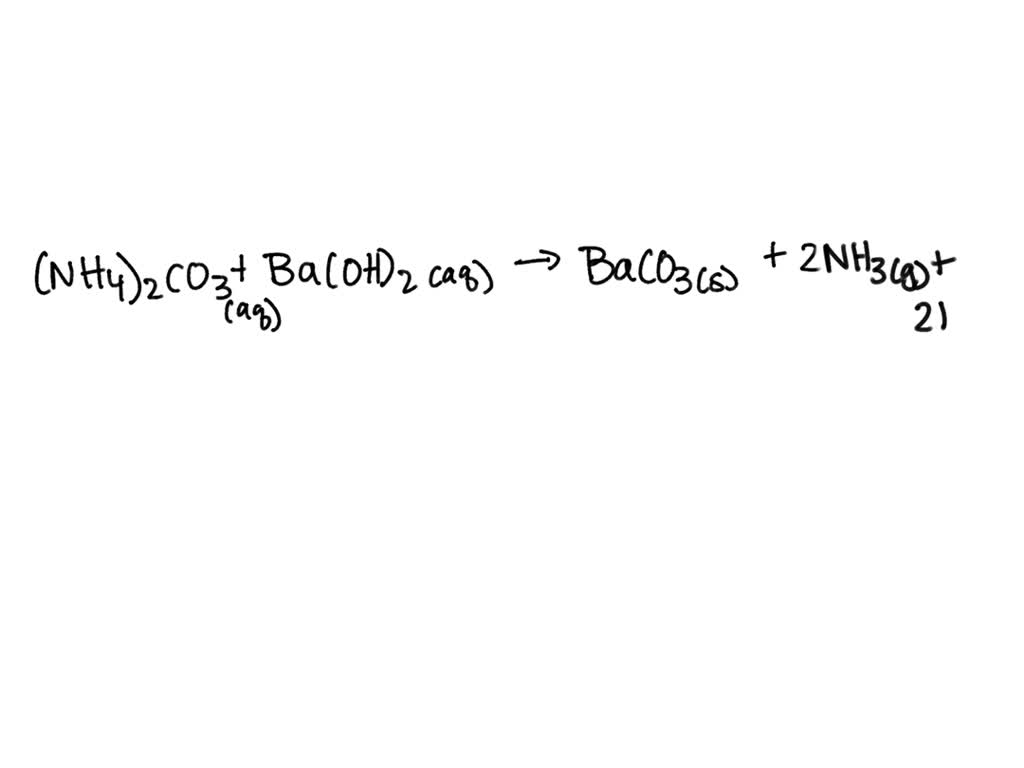
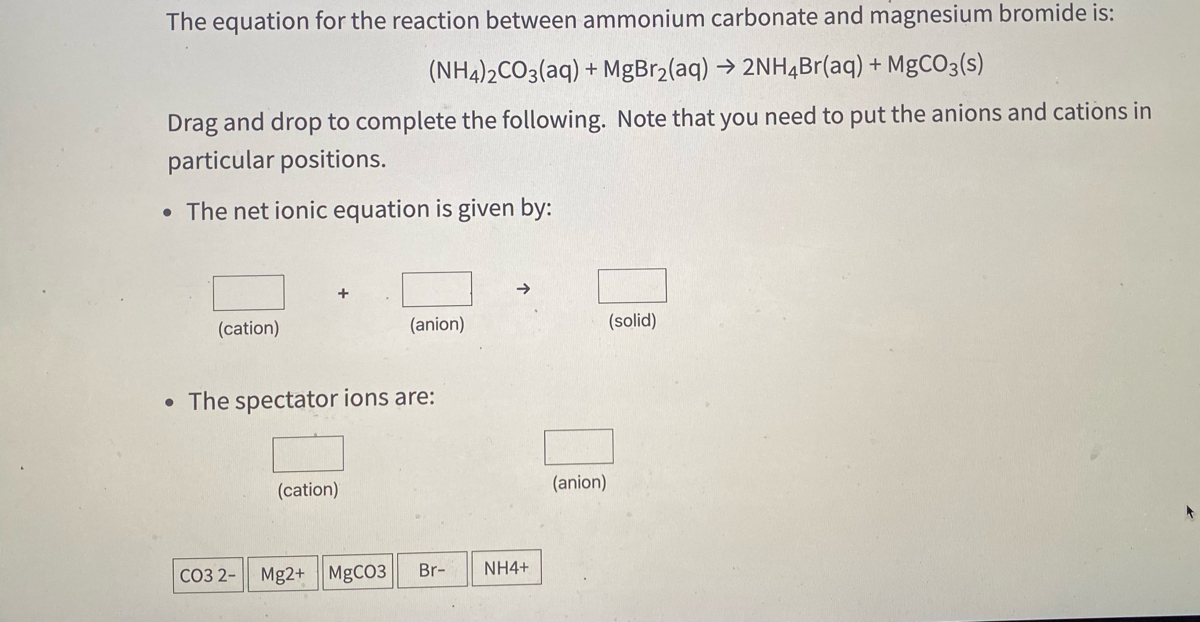
SOLVED: Which one of the following reaction equations is the net ionic equation for the reaction of ammonium carbonate solution with barium hydroxide solution? Group of answer choices 2NH4+(aq) + CO32-(aq) +
![PDF] Solubility behavior of rare earths with ammonium carbonate and ammonium carbonate plus ammonium hydroxide: Precipitation of their peroxicarbonates | Semantic Scholar PDF] Solubility behavior of rare earths with ammonium carbonate and ammonium carbonate plus ammonium hydroxide: Precipitation of their peroxicarbonates | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3de3b6bd91c18f480ee899ed67da03ed6b45e088/2-Table1-1.png)
PDF] Solubility behavior of rare earths with ammonium carbonate and ammonium carbonate plus ammonium hydroxide: Precipitation of their peroxicarbonates | Semantic Scholar

Ammonium Carbonate (506-87-6) ((NH4)2@CO3) - China Ammonium Carbonate, Baker's Ammonia | Made-in-China.com

Amazon.com: Spectrum A1154-500GM Ammonium Carbonate, Powder, Technical Grade, (NH4)2CO3, 10 cc : Industrial y Científico

What is the formula of the compound formed between the ammonium ion and the carbonate ion? A. NH4CO3 B. NH4(CO3)2 C. (NH4)2CO3 D. (NH4)3CO3 | Homework.Study.com

organic chemistry - reaction of diketone with ammonium carbonate at 100-115 degree celsius - Chemistry Stack Exchange

SOLVED: When ammonium carbonate is heated, three gases are produced by its decomposition: (NH4)2CO3 â†' 2 NH3 (g) + CO2 (g) + H2O (g). If 9.0 g of ammonium carbonate is decomposed

Dual Role of (NH4)2CO3 Enables Defluorinative Synthesis of β-Fluoroalkylated Aminovinyl Ketones | Organic Letters

![Ammonium Carbonate [(NH4)2CO3] Molecular Weight Calculation - Laboratory Notes Ammonium Carbonate [(NH4)2CO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/ammonium-carbonate-molecular-weight-calculation-300x198.jpg)








![Ammonium carbonate - Optional[FTIR] - Spectrum - SpectraBase Ammonium carbonate - Optional[FTIR] - Spectrum - SpectraBase](https://spectrabase.com/api/spectrum/I567hx3XQ8t/structure.png?h=300&w=382)

![ASAP] Primary Amination of Ar2P(O)H with (NH4)2CO3 as an Ammonia So ASAP] Primary Amination of Ar2P(O)H with (NH4)2CO3 as an Ammonia So](https://www.researcher-app.com/image/eyJ1cmkiOiJodHRwczovL3MzLWV1LXdlc3QtMS5hbWF6b25hd3MuY29tL3N0YWNrYWRlbWljL3Byb2R1Y3Rpb24vcGFwZXIvOTU4NjI5Mi5wbmciLCJmb3JtYXQiOiJ3ZWJwIiwicXVhbGl0eSI6MTAwLCJub0NhY2hlIjp0cnVlfQ==.webp)
![Solved 1. [20 points] When heated ammonium carbonate | Chegg.com Solved 1. [20 points] When heated ammonium carbonate | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F8e6%2F8e6a7178-f042-4c3b-a7c7-cb9d1e98dddb%2FphpYG52J9.png)