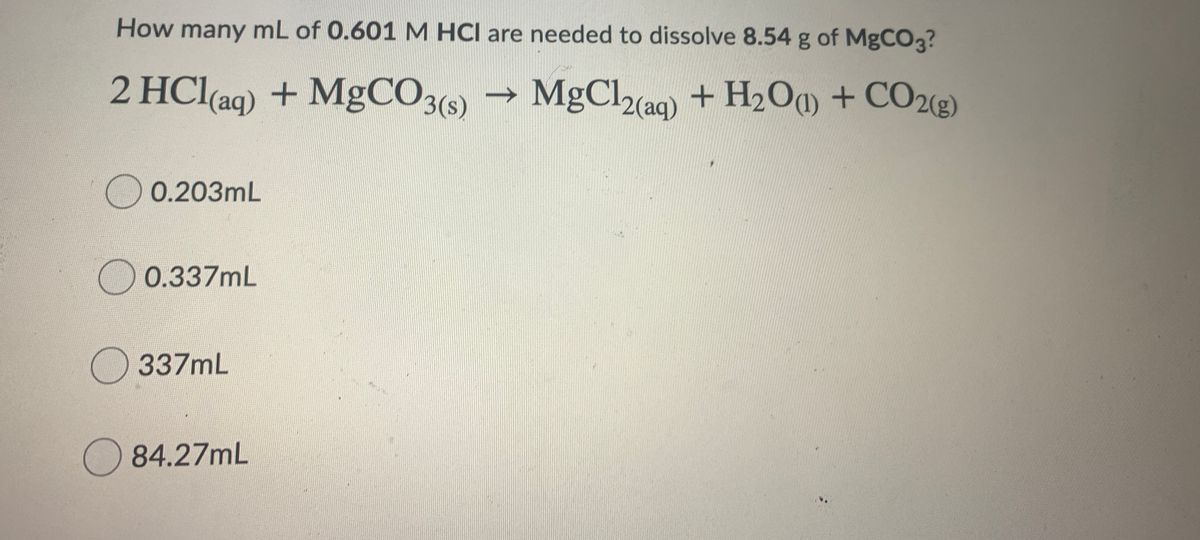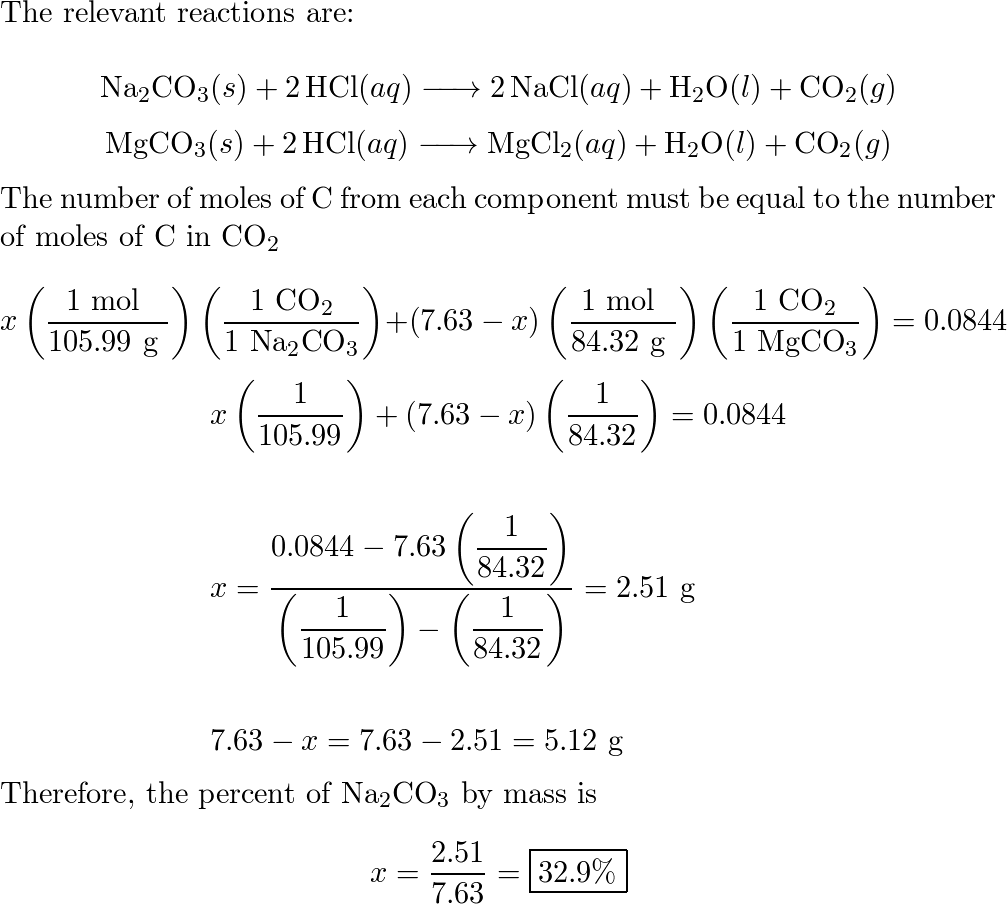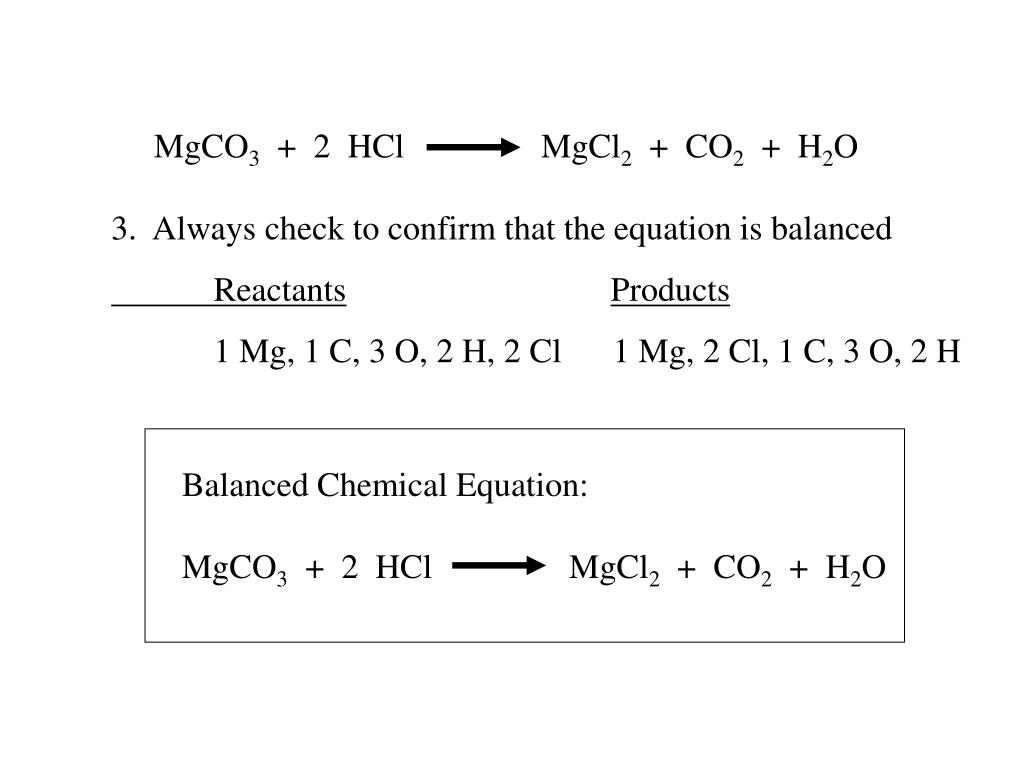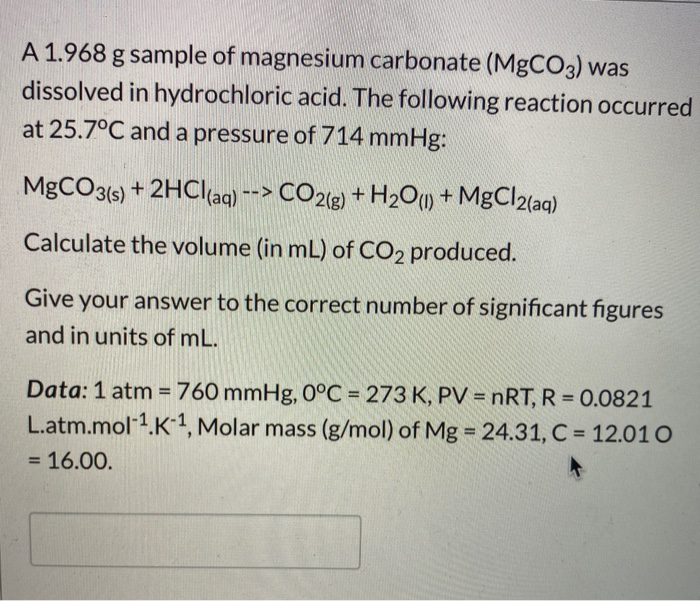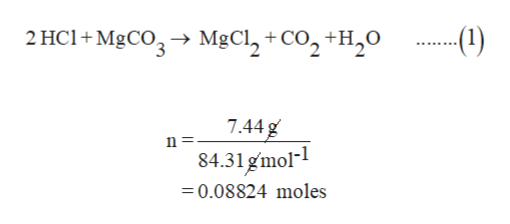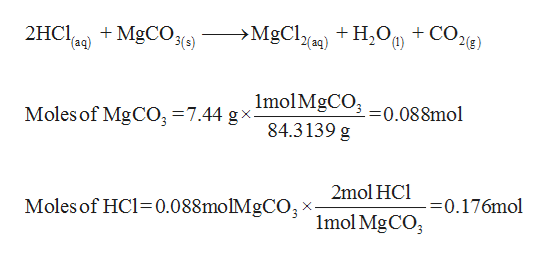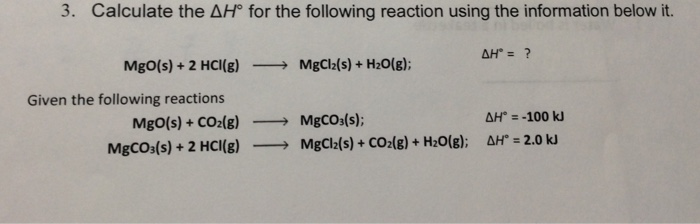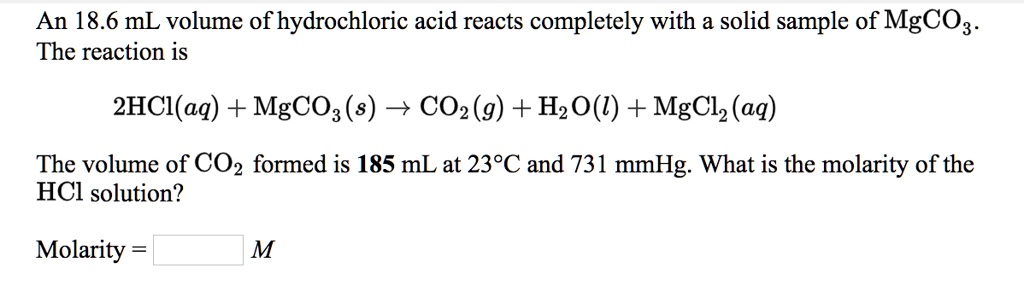
SOLVED: An 18.6 mL volume of hydrochloric acid reacts completely with a solid sample of MgCO3. The reaction is: 2HCl(aq) + MgCO3(s) = CO2(g) + H2O(l) + MgCl2(aq) The volume of CO2

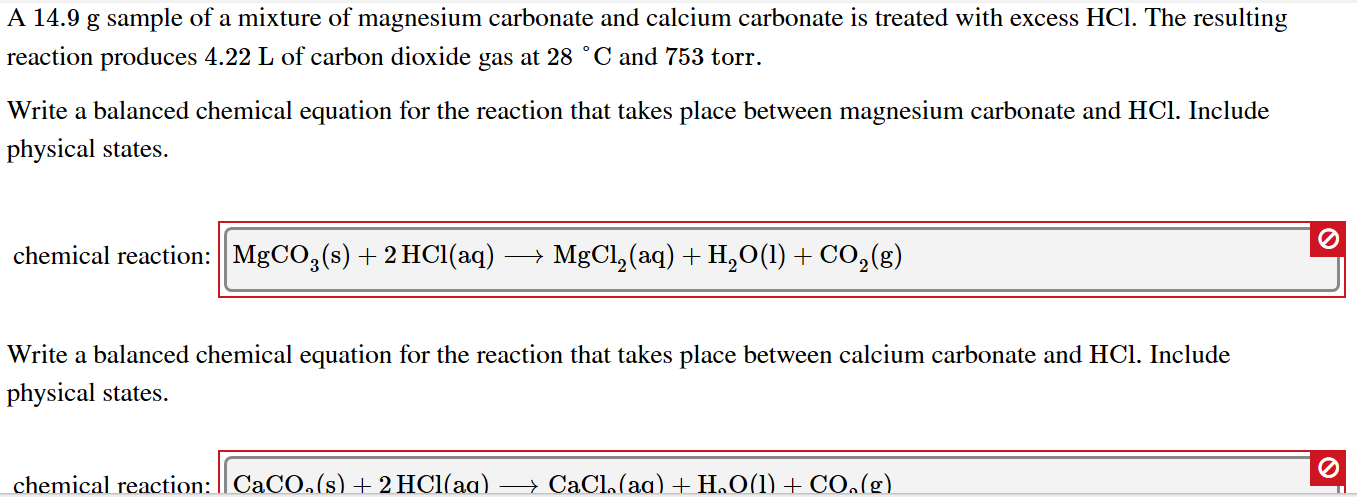
SOLVED:Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction that occurs
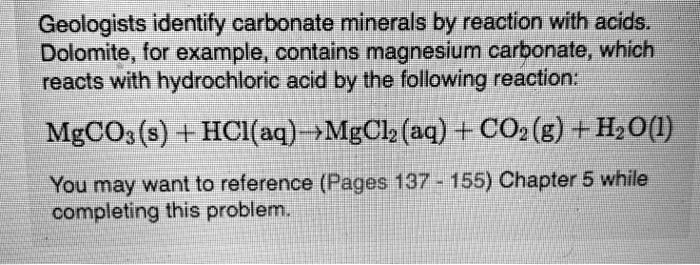
SOLVED: Geologists identify carbonate minerals by reaction with acids. Dolomite, for example, contains magnesium carbonate, which reacts with hydrochloric acid by the following reaction: MgCO3 (s) + HCl (aq) â†' MgCl2 (aq) +