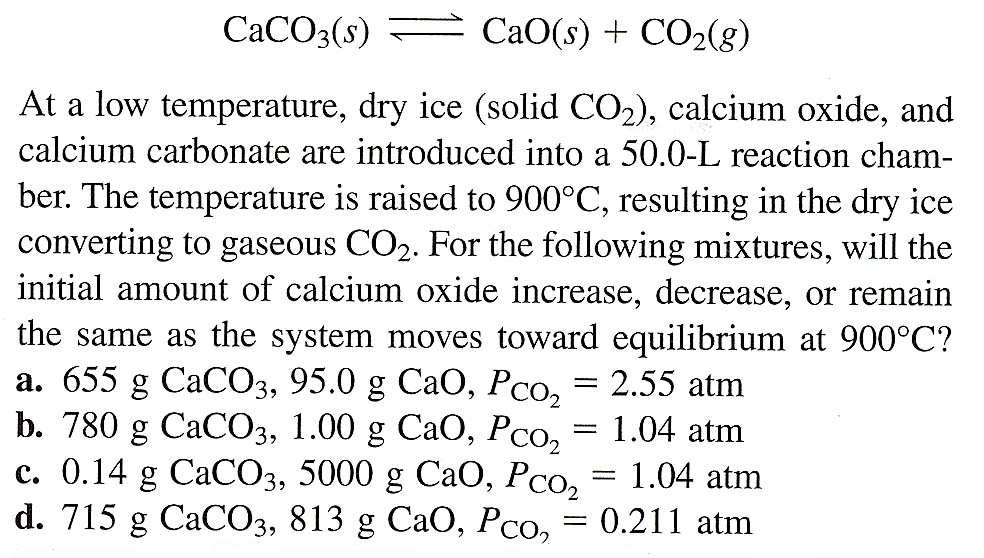Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
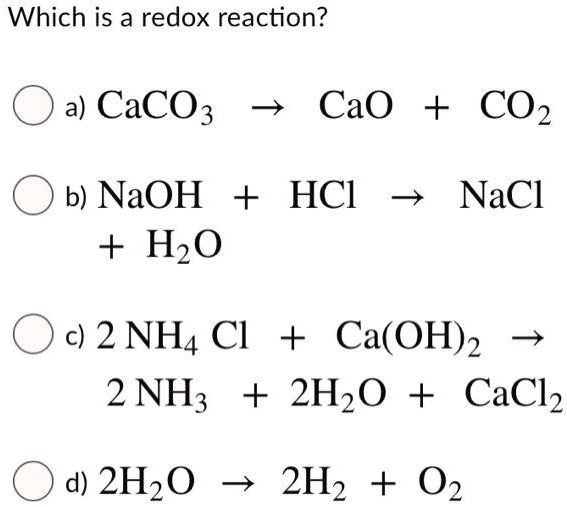
SOLVED: Which is a redox reaction? a) CaCO3 â†' CaO + CO2 b) NaOH + H2O â†' HCl + NaCl c) 2 NH4Cl + Ca(OH)2 â†' 2 NH3 + 2H2O + CaCl2 d) 2H2O â†' 2H2 + O2

Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram
In the equilibrium CaCO3(s)Cao (s)+CO2(g) at 1073 K, the pressure of CO2 isfound to be 2.5 x 10 Pa. The equilibriumconstant for the reaction at 1073 K will be.(a) 0.25(c)25(b) 2.5(d) 250

When calcium carbonate (CaCO3) is heated, it decomposes to form calcium oxide (CaO) and carbon dioxide - Brainly.com

How to Balance Ca(HCO3)2 = CaCO3 + CO2 + H2O (Decomposition of Calcium hydrogen carbonate) - YouTube

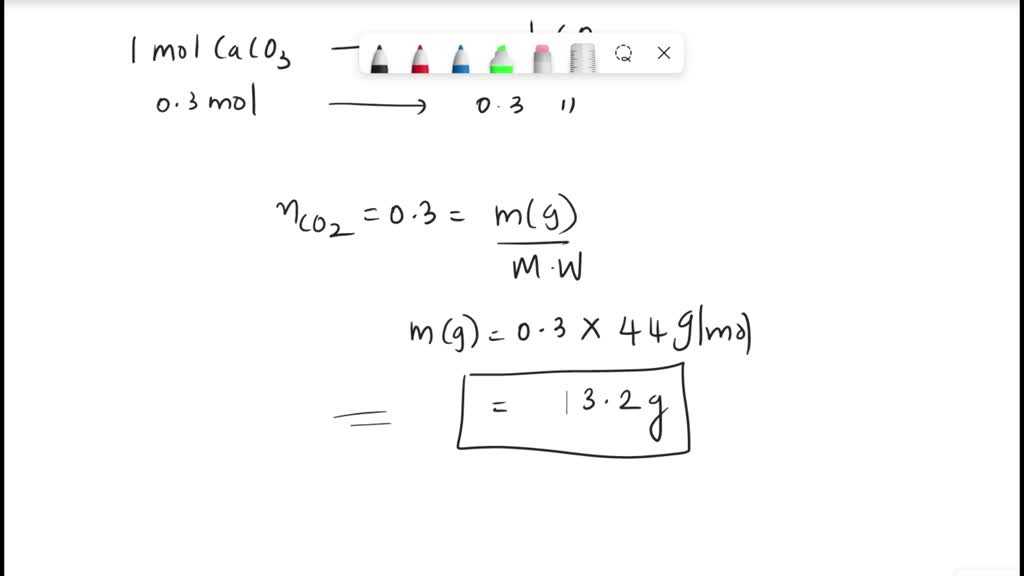
How many grams of CO2 would be required to be passed through a tank of lime water to produce 100 grams of CaCO3?

Reaction steps involved in the formation of the CaCO3 sol from calcium... | Download Scientific Diagram


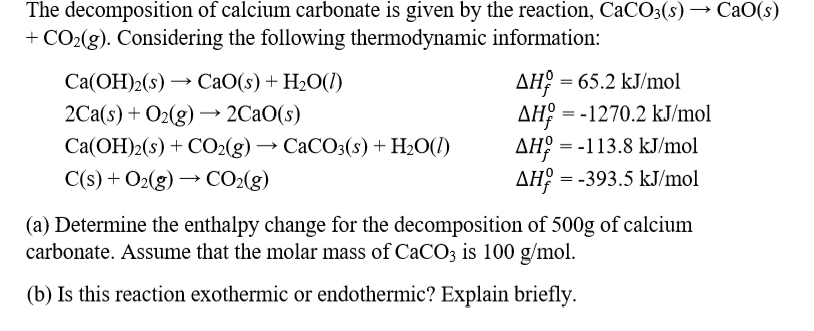






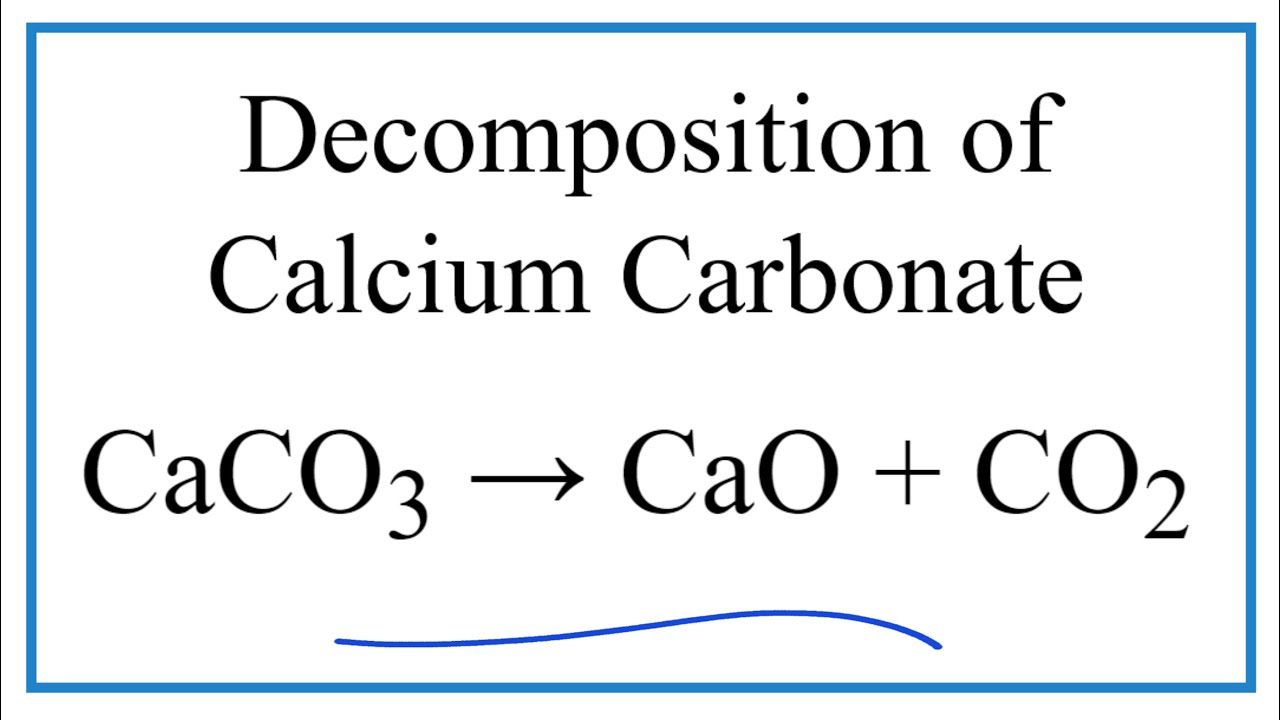
![ANSWERED] Given the following chemical equation CaO... - Physical Chemistry ANSWERED] Given the following chemical equation CaO... - Physical Chemistry](https://media.kunduz.com/media/sug-question-candidate/20220517131515311837-4392391.jpg)