CaCO3(s) → ← CaO(s) + CO2(g) When heated, calcium carbonate decomposes according to the equation above. In a study of the
30. Calcium carbonate reacts with HCl to give CaCl,and CO2 according to the reaction:+ 2HCI(aq) CaCl, (aq) + CO2(g)CaCO3(s)H20(I) What mass of 20

X-ray diffraction patterns of CaCO3(s) particles. The indexes c and v... | Download Scientific Diagram
The partial pressure of carbon dioxide in the reaction CaCO3(s) ⇌ CaO(s) + CO2(g) is 1.017 x 10^-3 atm at 500° C. - Sarthaks eConnect | Largest Online Education Community

OneClass: 4. Calculate ΔGo for the reaction: CaCO3(s) â†' CaO(s) + CO2(g), given ΔGfo CO2(g) = -394...
CaCO3(s) → ← CaO(s) + CO2(g) When heated, calcium carbonate decomposes according to the equation above. In a study of the

For CaCO3(s) -- CaO(s) +CO2(g) at 977 C , H = 174 KJ / mol Then find - Chemistry - Practical Work - 13011695 | Meritnation.com
CaO(s) + CO2(g) → CaCO3(s) + heat What is the total mass of CO2(s) needed to produce 300. grams of CaCO3(s)? - Quora
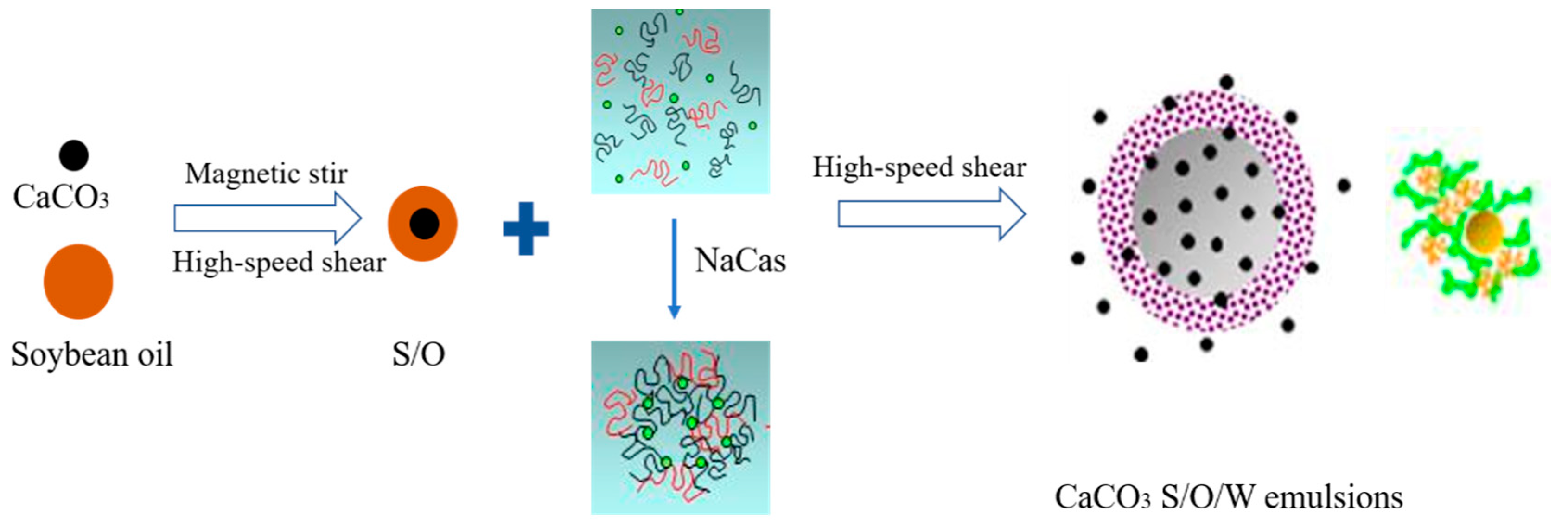
Foods | Free Full-Text | Stability, Microstructure, and Rheological Properties of CaCO3 S/O/W Calcium-Lipid Emulsions






![ANSWERED] Given the following chemical equation CaO... - Physical Chemistry ANSWERED] Given the following chemical equation CaO... - Physical Chemistry](https://media.kunduz.com/media/sug-question-candidate/20220517131515311837-4392391.jpg)