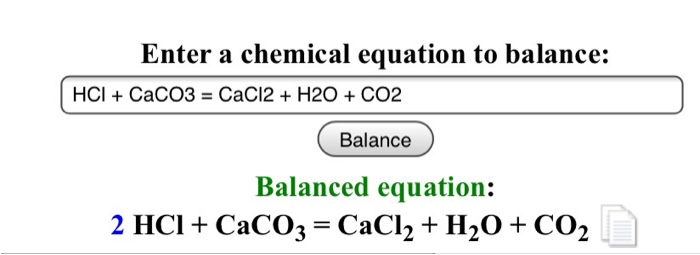
How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O | How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O Hey there! Are you struggling with balancing
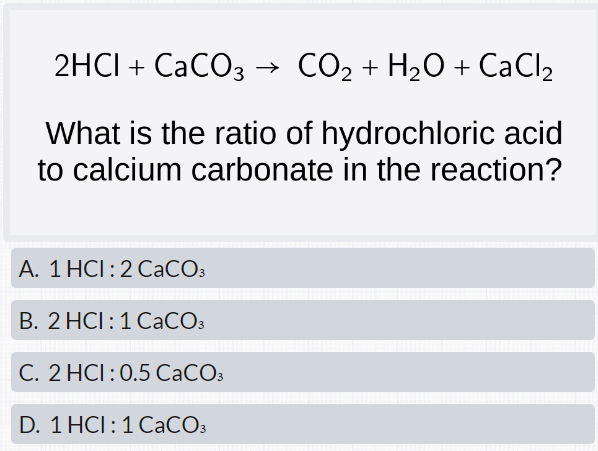
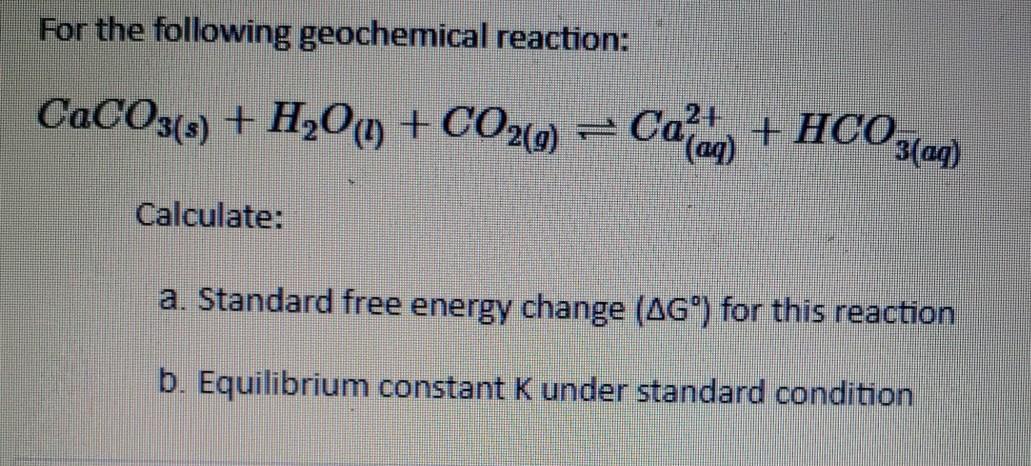
The mass of CaCO3 required to react completely with 20 mL of 1.0 M HCL as per the reaction CaCO3+2HCl–>CaCl2+CO2+H2O

Figure 1 from DFT Calculations with van der Waals Interactions of Hydrated Calcium Carbonate Crystals CaCO3·(H2O, 6H2O): Structural, Electronic, Optical, and Vibrational Properties. | Semantic Scholar

Examining the Accuracy of Density Functional Theory for Predicting the Thermodynamics of Water Incorporation into Minerals: The Hydrates of Calcium Carbonate | The Journal of Physical Chemistry C

100 g CaCo3 reactwith20g HCl according tobfollowing equation CaCo3 +2HCl=CaCl2+H2o+Co2 What is mass of Co2 willbeproduced - Chemistry - Some Basic Concepts of Chemistry - 12831553 | Meritnation.com
What is the balanced chemical equation for when a nitric acid and calcium carbonate react to salt, water, and carbon dioxide? - Quora

SOLVED: Enthalpy related question here. Solid calcium carbonate reacts with acid to release CO2 as shown in the reaction below: CaCO3 (s) + 2HCl â†' CaCl2 (aq) + H2O (l) + CO2 (