40. Consider the reaction CaCO3+2HCL (l) 》CaCl2+CO2+H2O (l).what mass of CaCO3 is required to react with 20mL 1M HCL?

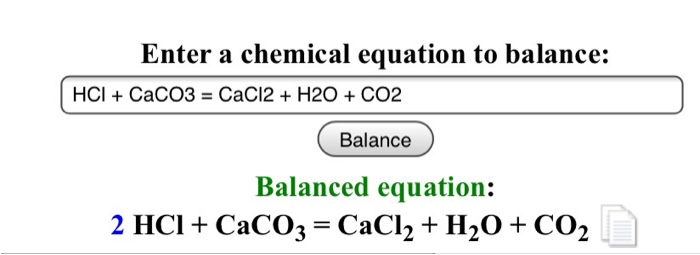
HCl+CaCO3=CaCl2+H2O+CO2 balance the chemical equation @mydocumentary838. hcl+caco3=cacl2+h2o+co2 - YouTube
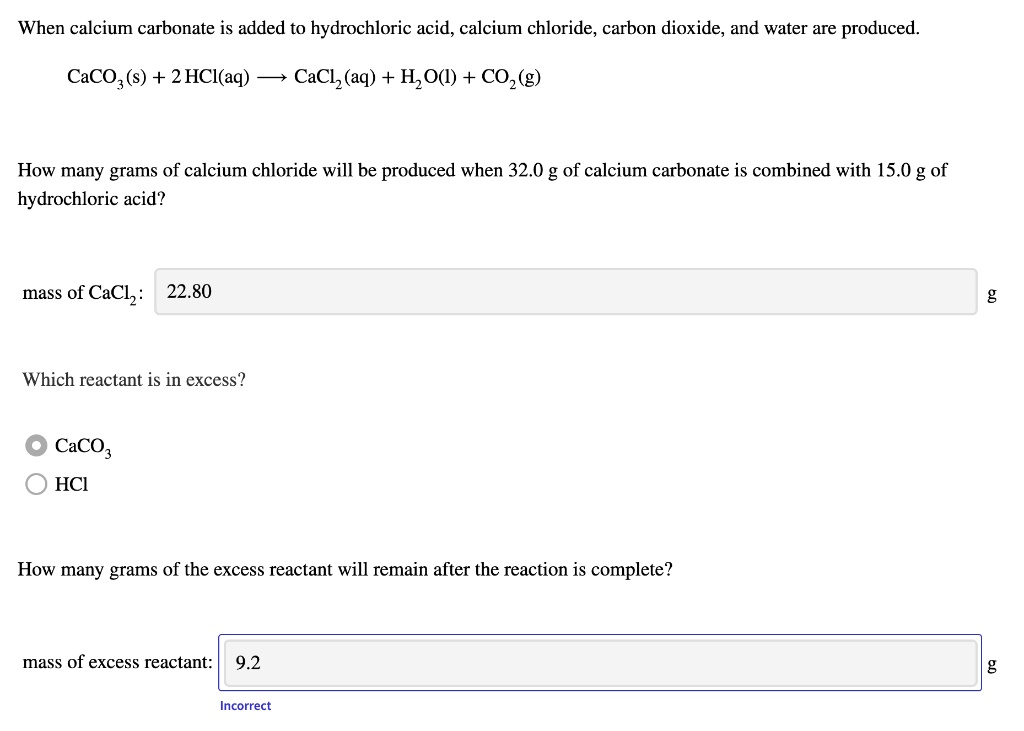
SOLVED: When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced. CaCO3(s) + 2 HCl(aq) â†' CaCl2(aq) + H2O(l) + CO2(g) How many grams of calcium

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

Calcium carbonate reacts with aqueous HCI to give CaCl2 and CO2 according to the reactions: CaCO3 (s) + 2HCl (aq)→ CaCl2 (aq) + CO2 (g) + H2O What mass of CaCO3 is
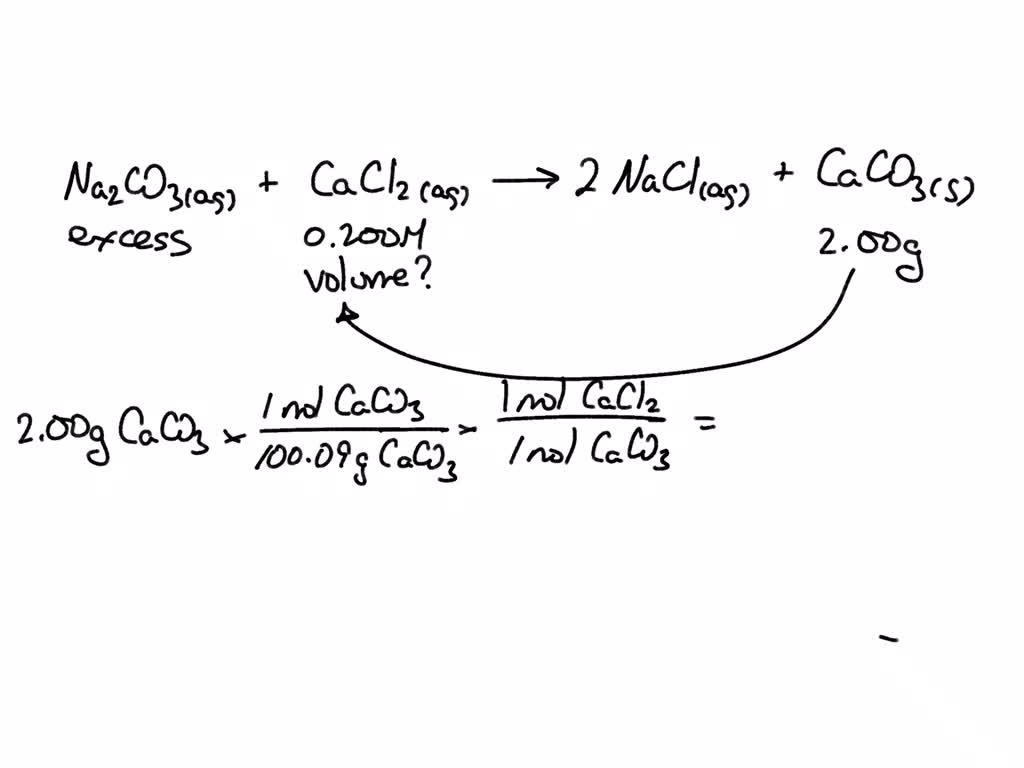
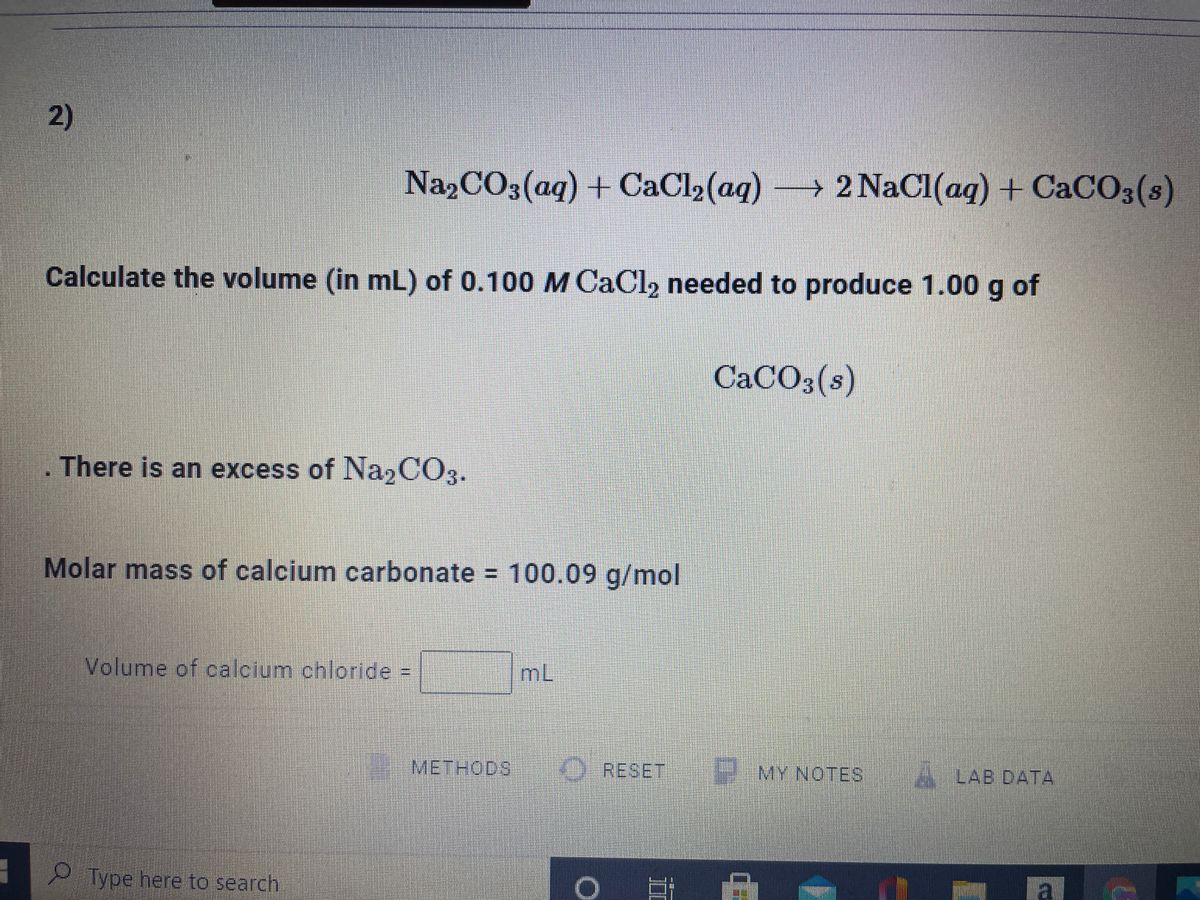
SOLVED: Na2CO3(aq) + CaCl2(aq) ——-> 2 NaCl(aq) +CaCO3(s) Calcuate the volume (in mL) of 0.200 M CaCl2 needed to produce 2.00g of CaCO3(s). There is an excess of Na2CO3 and the molar

Preparation of SMA/CaCO3/CaCl2@SiO2 as a Fluid Loss Agent Based on PSA/Ca-MMT/CaCl2@SiO2 | American Laboratory

CaCO3 + 2HCl → CaCl2 + H2O + CO2 The mass of calcium chloride formed when 2.5 g of calcium carbonate is dissolved in excess of hydrochloric acid is:
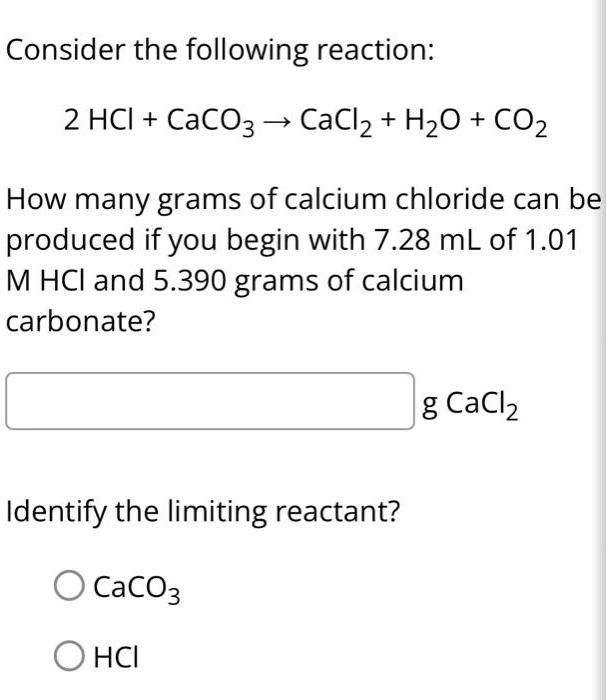
SOLVED: Consider the following reaction: 2 HCl + CaCO3 -> CaCl2 + H2O + CO2 How many grams of calcium chloride can be produced if you begin with 7.28 mL of 1.01




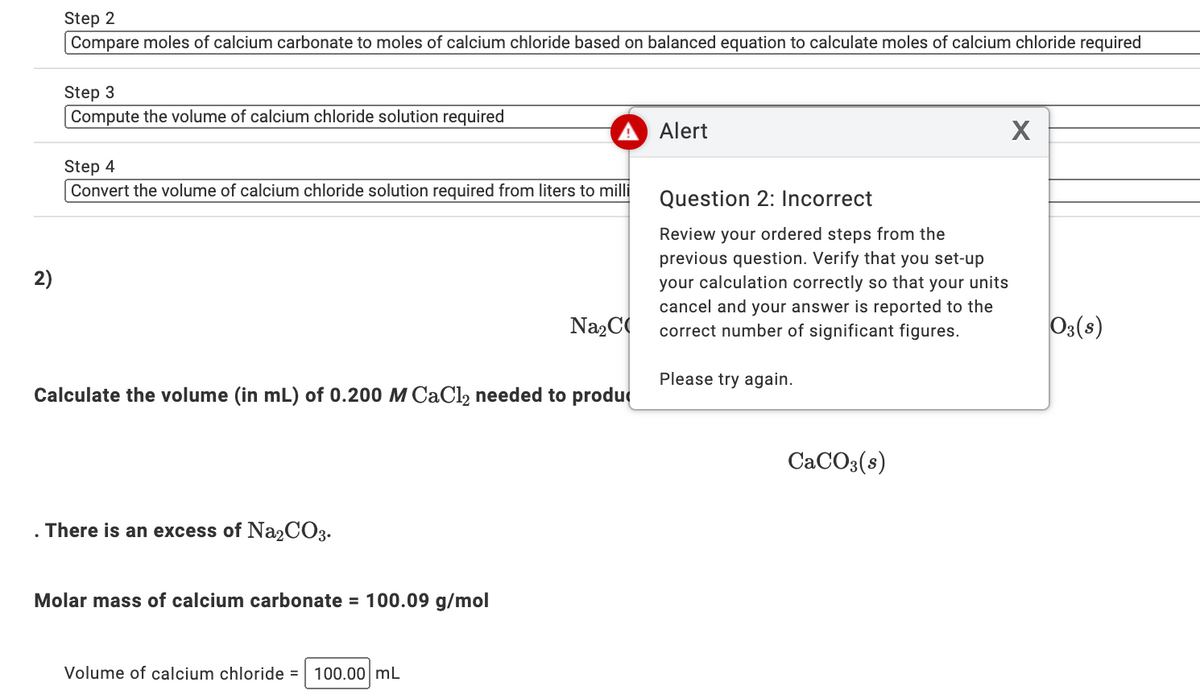
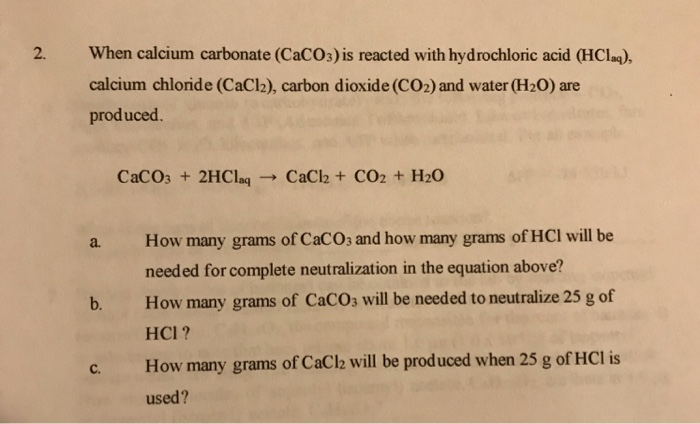

![ANSWERED] Na₂CO3(aq) +CaCl₂(aq) -> 2 NaCl(aq) + CaCO... - Organic Chemistry ANSWERED] Na₂CO3(aq) +CaCl₂(aq) -> 2 NaCl(aq) + CaCO... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/61173905-1656941477.49281.jpeg)

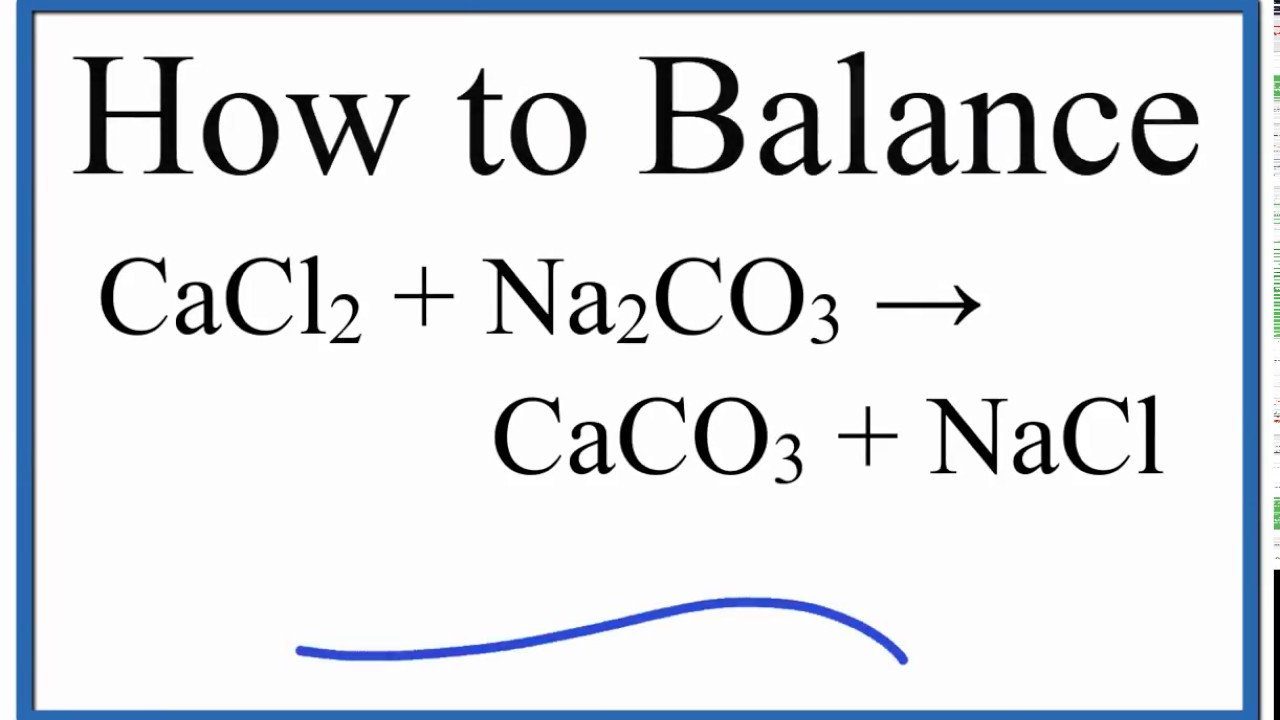

![ANSWERED] When calcium carbonate is added to hydroc... - Physical Chemistry ANSWERED] When calcium carbonate is added to hydroc... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/53143896-1659267628.173333.jpeg)