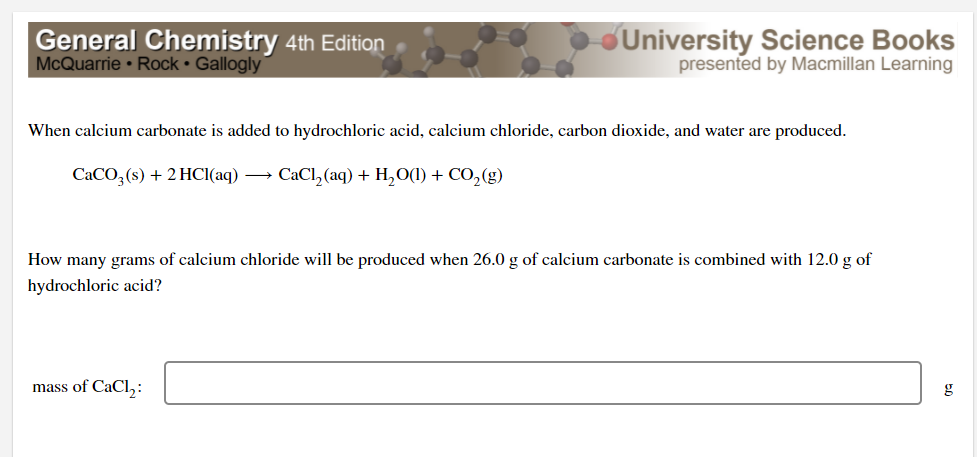
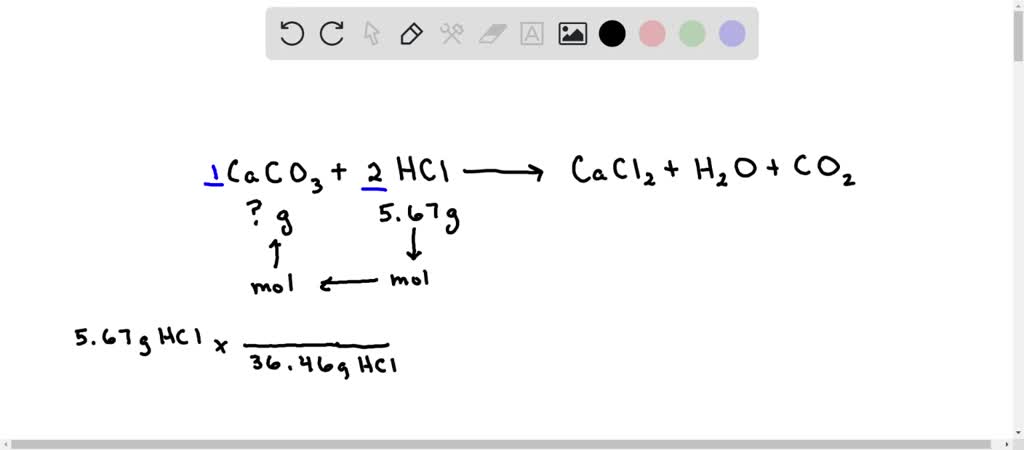
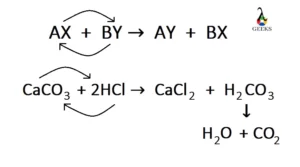16. In a chemical reaction, caco3+2hcl= cacl2 +co2+h2o. 25ml hcl and 0.75M Calculate the amount of caco3
SOLVED: Calcium carbonate (CaCO3) is a common ingredient in antacids. It works by neutralizing the hydrochloric acid in your stomach according to the following reaction: CaCO3 + 2HCl â†' CaCl2 + H2O +

SOLVED: If 2.5g of CaCO3 is mixed with 2.0 g of HCl to complete the reaction CaCO3 + 2HCl ——–> CaCl2 +CO2 +H2O, What is limiting and what amount of CO2 will
Complete and balance the following chemical equations: i. CaCO3 + HCl - Sarthaks eConnect | Largest Online Education Community
40. Consider the reaction CaCO3+2HCL (l) 》CaCl2+CO2+H2O (l).what mass of CaCO3 is required to react with 20mL 1M HCL?

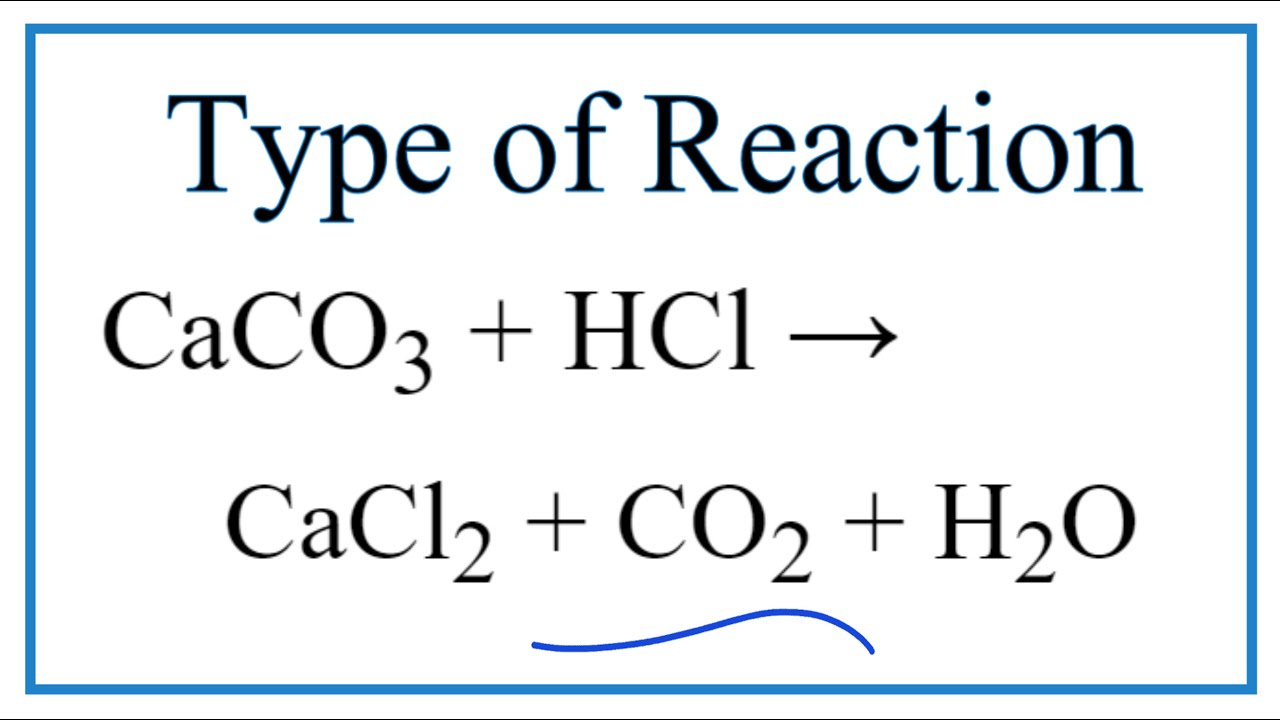
How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O | How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O Hey there! Are you struggling with balancing
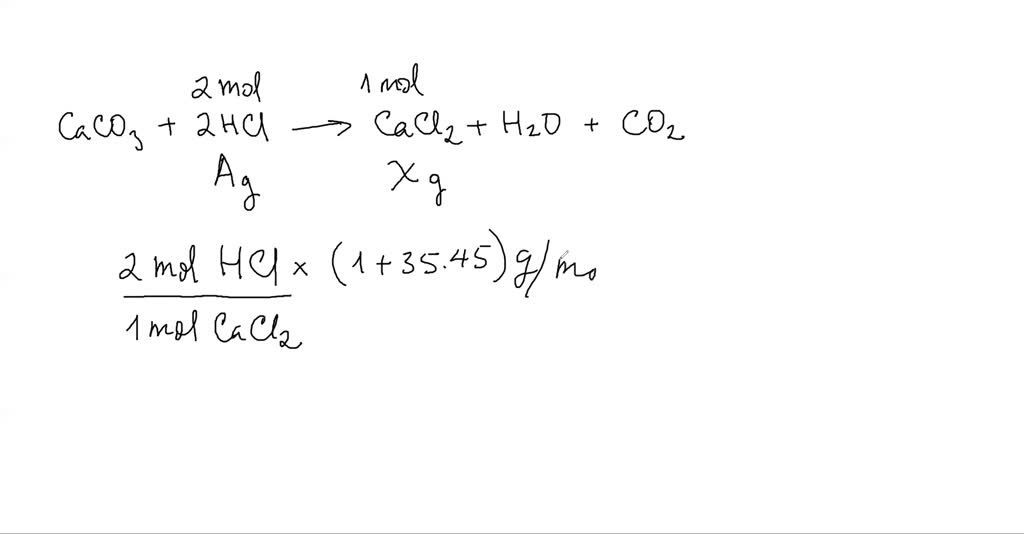
SOLVED:Design a concept map for the following reaction. CaCO3(s)+2 HCl(aq) →CaC2(aq)+H2O(𝔩)+CO2(g) The concept map should explain how to determine the mass of CaCl2 produced from a given mass of HCl.



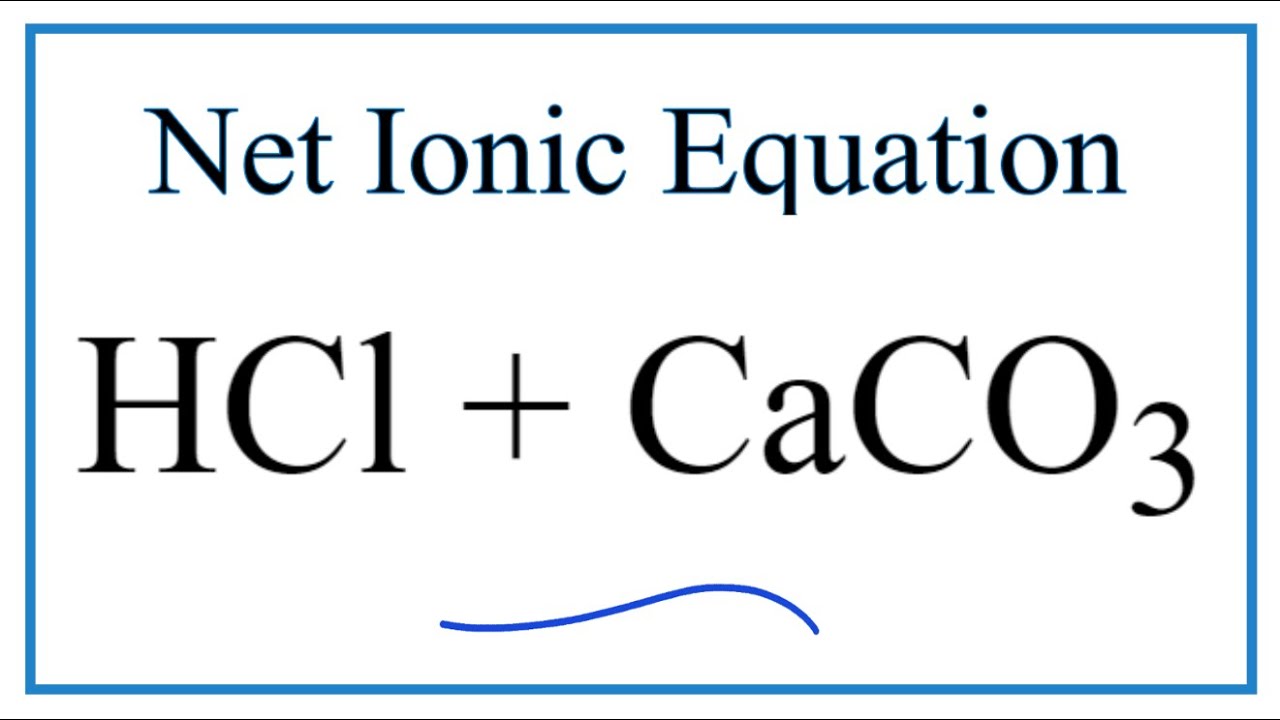


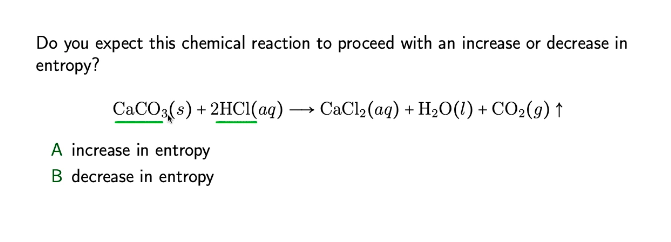
![Explain the reaction. CaCO3 + 2HCl⟶CaCl2 + H2O + CO2 [g ] Explain the reaction. CaCO3 + 2HCl⟶CaCl2 + H2O + CO2 [g ]](https://haygot.s3.amazonaws.com/questions/1352729_1287783_ans_adc66c29654147fb965de67e86f7f9a2.jpeg)