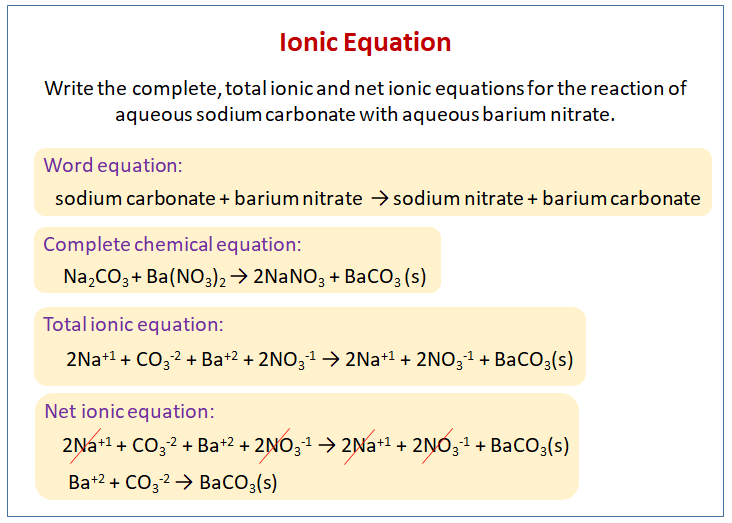
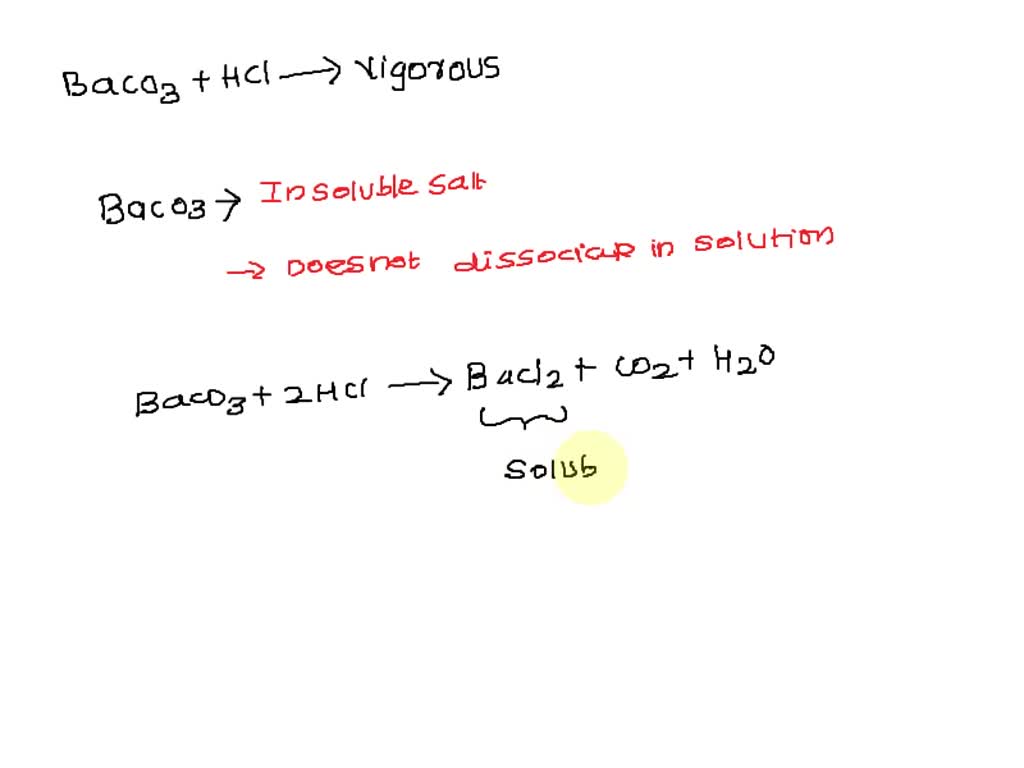
SOLVED: Write the net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and barium carbonate are combined. Write the net ionic equation for the reaction that occurs when

SOLVED: An ore of barium contains BaCO3. A 1.620 g sample of the ore was treated with HCl to dissolve the BaCO3. The resulting solution was filtered to remove insoluble material and

Géologie du Maroc / Geology of Morocco - The #Acid_Test for #Carbonate_Minerals : To most geologists, the term "acid test" means placing a drop of dilute (5% to 10%) hydrochloric acid on

The experimental enthalpies of solution of Y, BaCO3 and CoCl2·4.24H2O... | Download Scientific Diagram

CO2 capture via barium carbonate formation after its absorption with ammonia in a pilot scale column - ScienceDirect






![Barium Carbonate [BaCO3] Molecular Weight Calculation - Laboratory Notes Barium Carbonate [BaCO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/barium-carbonate-molecular-weight-calculation-300x205.jpg)


![LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes – Master Organic Chemistry LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes – Master Organic Chemistry](https://cdn.masterorganicchemistry.com/wp-content/uploads/2022/11/0-LiAlHOtBu3-selective-reagent-for-reduction-of-acid-halides-to-aldehydes.gif)