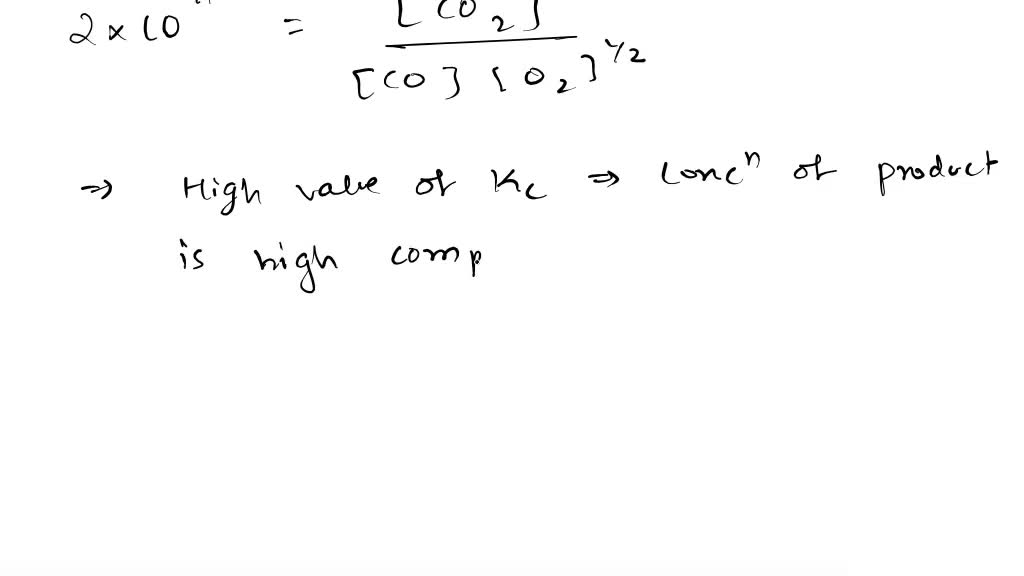Carbon Monoxide Co And Carbon Dioxide Co2 Molecule Structural Chemical Formula And Molecule Model Stock Illustration - Download Image Now - iStock
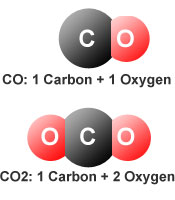
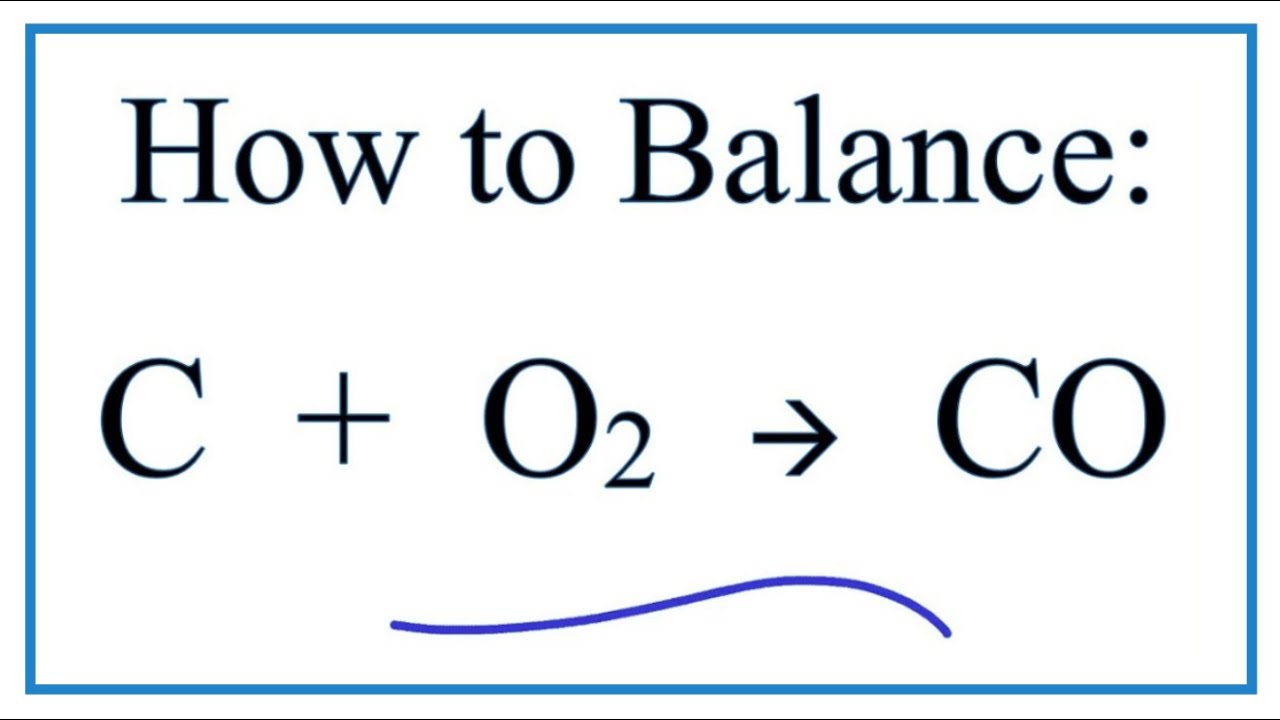
co+o2 gives co2 and c+o2 also give co2 in both cases there is difference in reaction then how product is same
![CO2 = CO + [O]: recent advances in carbonylation of C–H bonds with CO2 - Chemical Communications (RSC Publishing) CO2 = CO + [O]: recent advances in carbonylation of C–H bonds with CO2 - Chemical Communications (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/D0CC00547A)
CO2 = CO + [O]: recent advances in carbonylation of C–H bonds with CO2 - Chemical Communications (RSC Publishing)

Chemical Equations Section 7.2 & 7.3. Chemical Equations CH 4 (g) + O 2(g) CO 2 (g) + H 2 O (g) Reactantsproducts Means to produce solid (s) Liquid. - ppt download
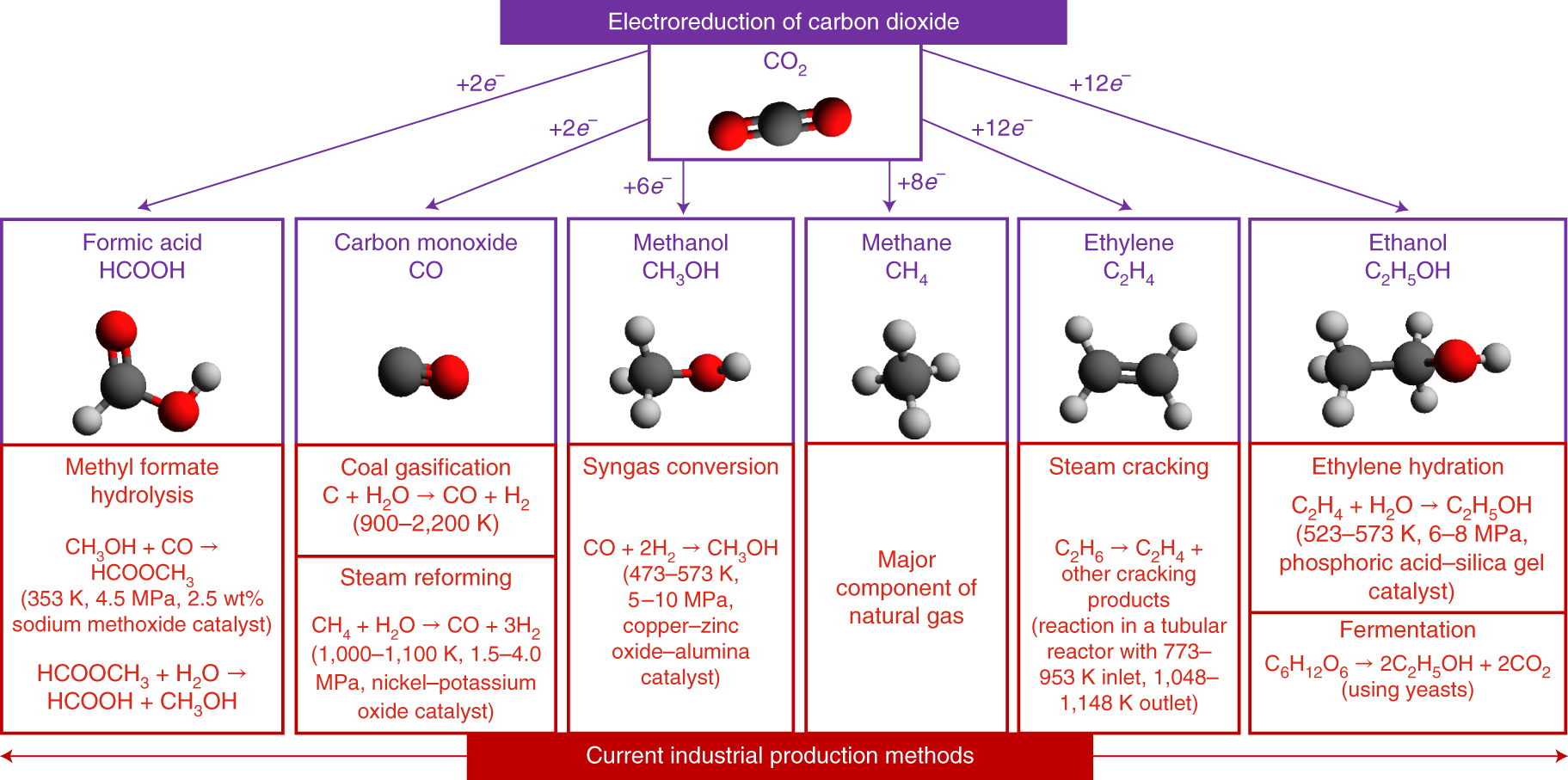
Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption | Nature Energy
Given that, C + O2 → CO2; ΔH° = -x kJ, 2CO + O2 → 2CO2; ΔH° = -y kJ. The enthalpy of formation of CO will be - Sarthaks eConnect | Largest Online Education Community
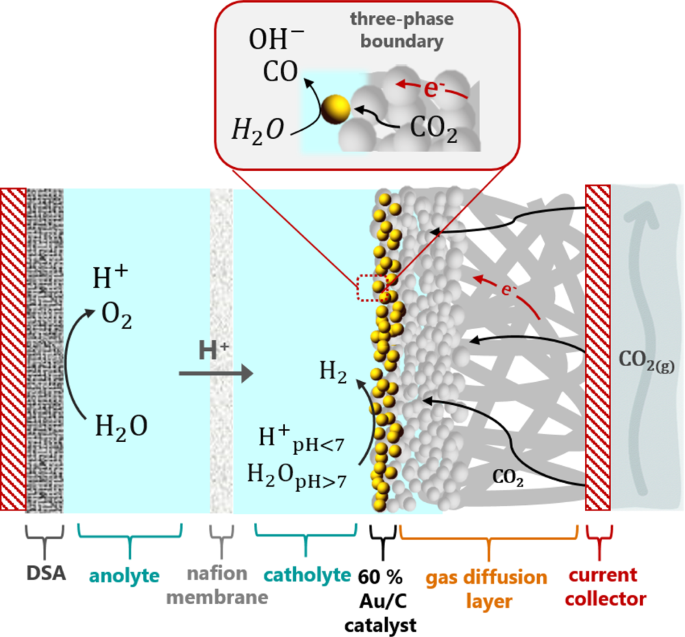
Efficiency and selectivity of CO2 reduction to CO on gold gas diffusion electrodes in acidic media | Nature Communications